Lithium Harvesting from Sea Water

Lithium is the most used essential component of batteries and therefore electric cars. However lithium as resource is limited and must be mined under difficult conditions which destroys environment and creates toxic pollution. A research team in Saudi-Arabia claims to have found a way to yield lithium from sea water.
Students of the saudi-arabic King-Abdullah Unversity of Science and Technology (KAUST) developed an electrochemical cell that can filter lithium out of sea water. This research project has a serious background. It is estimated that until 2080 all land reserves of lithium may exhaust because of the increased demand. An alternative must be found.
The lithium content of the sea is 5000 times higher than land. The problem: The lithium concentration in the sea is with 0.2 ppm (parts per million) extremely low. With the electrochemical cell it is possible increase the concentration by multiple times. What is special about the cell is a ceramic membrane made of Lanthan-Titan-Oxid (LLTO). Its crystal structure contains holes which are just big enough to let lithium ions through. All other metal ions are blocked.
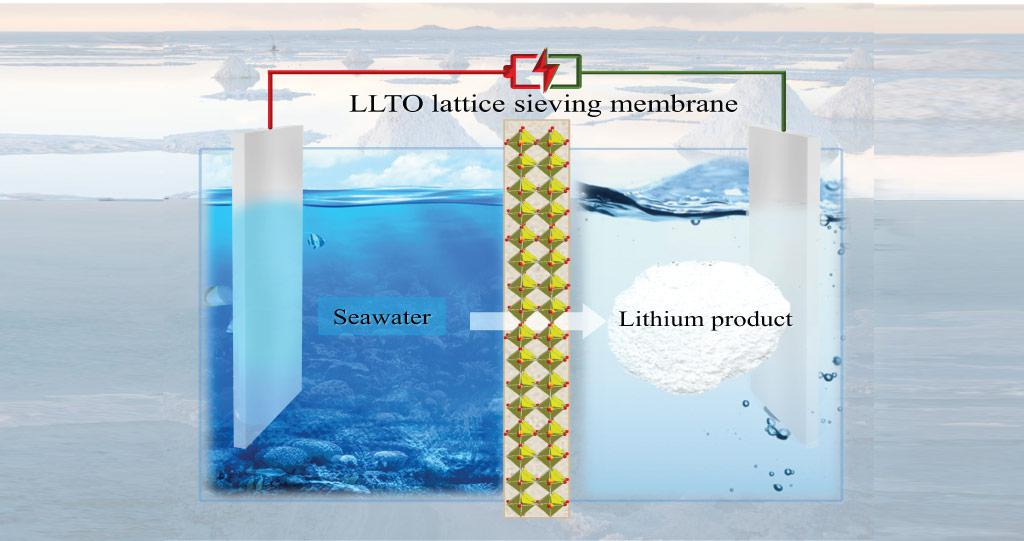
The developer of the cell, Zhen Li, states that never even before have LLTO-membrane be used to extract and enrich lithium ions.
The manufacturing process is however difficult. After the lithium has penetrated the membrane, four further enrichment cycles are required in order to enrich the lithium concentration to above 9000 ppm. This would be enough to fulfill requirements of battery producers.
For the experiment student tested water from the red sea. One advantage of the method is critical. According to estimation the cell requires about 4 euros of electricity in order to yield one kilogram of lithium from sea water.
The group leader Zhiping Lay states that they are working to optimize the membrane structure and the design of the cells for increasing the efficiency.
The method could be promising if it does not pollute the ocean nor affects the flora and fauna of the sea.
Reference:
Lithium Harvesting from Sea Water =================================  Lithium is the most used essential component of batteries and therefore electric cars. However lithium as resource is limited and must be mined under difficult conditions which destroys environment and creates toxic pollution. A research team in Saudi-Arabia claims to have found a way to yield lithium from sea water. Students of the saudi-arabic King-Abdullah Unversity of Science and Technology (KAUST) developed an electrochemical cell that can filter lithium out of sea water. This research project has a serious background. It is estimated that until 2080 all land reserves of lithium may exhaust because of the increased demand. An alternative must be found. The lithium content of the sea is 5000 times higher than land. The problem: The lithium concentration in the sea is with 0.2 ppm (parts per million) extremely low. With the electrochemical cell it is possible increase the concentration by multiple times. What is special about the cell is a ceramic membrane made of Lanthan-Titan-Oxid (LLTO). Its crystal structure contains holes which are just big enough to let lithium ions through. All other metal ions are blocked.  The developer of the cell, Zhen Li, states that never even before have LLTO-membrane be used to extract and enrich lithium ions. The manufacturing process is however difficult. After the lithium has penetrated the membrane, four further enrichment cycles are required in order to enrich the lithium concentration to above 9000 ppm. This would be enough to fulfill requirements of battery producers. For the experiment student tested water from the red sea. One advantage of the method is critical. According to estimation the cell requires about 4 euros of electricity in order to yield one kilogram of lithium from sea water. The group leader Zhiping Lay states that they are working to optimize the membrane structure and the design of the cells for increasing the efficiency. The method could be promising if it does not pollute the ocean nor affects the flora and fauna of the sea. **Reference:** [Electrochemical cell harvests lithium from seawater](https://discovery.kaust.edu.sa/en/article/1133/electrochemical-cell-harvests-lithium-from-seawater)

